Decoding Digestive Dysfunctions
Hidden neural circuits controlling digestion
Late in 2024, I came across a piece of news from Caltech, where I did my undergraduate studies, about autonomic neurons and how they affect the digestive system. Shortly thereafter, during a social call with
, I learned that he’d taken note of the same article along with several other papers about the lower brain and nervous system. As another person with similar autonomic disorders, Vijay had connected the dots between some of our symptoms and these papers in a way that made sense to me, and after some discussion, we agreed that not enough attention is given to this kind of neuroscience.It’s important to remember that not all neural functions reside in the brain and spinal cord. We have a second nervous system called the enteric nervous system that controls digestion. Back in 2019, I started to develop chronic constipation, a condition that sounds relatively benign, but can lead to painful bloating, cramps, and more serious conditions like diverticulitis or fecal impaction. At the time, I was able to manage it through dietary changes and increased exercise, but then in 2021, I developed ME/CFS as a result of COVID-19, and any real physical activity became impossible. Needless to say, my gut became quite unhappy.
Another common gut dysfunction is Irritable Bowel Syndrome, which causes chronic bloating, diarrhea, constipation, and abdominal pain. As with other complex and systemic medical issues, the etiology of IBS and similar disorders is poorly understood and attributed to a wide range of possibilities. Therapeutic options are limited to lifestyle changes or medications that address specific symptoms, but because of the varied nature of these disorders, treatments are often unsuccessful.
Research on the enteric nervous system is essential for understanding many of the functions that underlie gut disorders like IBS. The macro physiological structures are well known, but there remains much to learn about the cellular-level details, especially in terms of enteroendocrine cells, which form synaptic connections to nerves in the digestive tract. These nerves originate from the spinal cord dorsal root ganglia as well as the vagal nodose ganglia, both of which play roles in the lower nervous system. Recent advances in neuroscience techniques are providing more insight into the workings of this “second brain” and one of its key players, the enterochromaffin cell (EC), a subset of enteroendocrine cells. These cells produce serotonin and regulate a variety of gut functions, including motility, secretion, and pain.
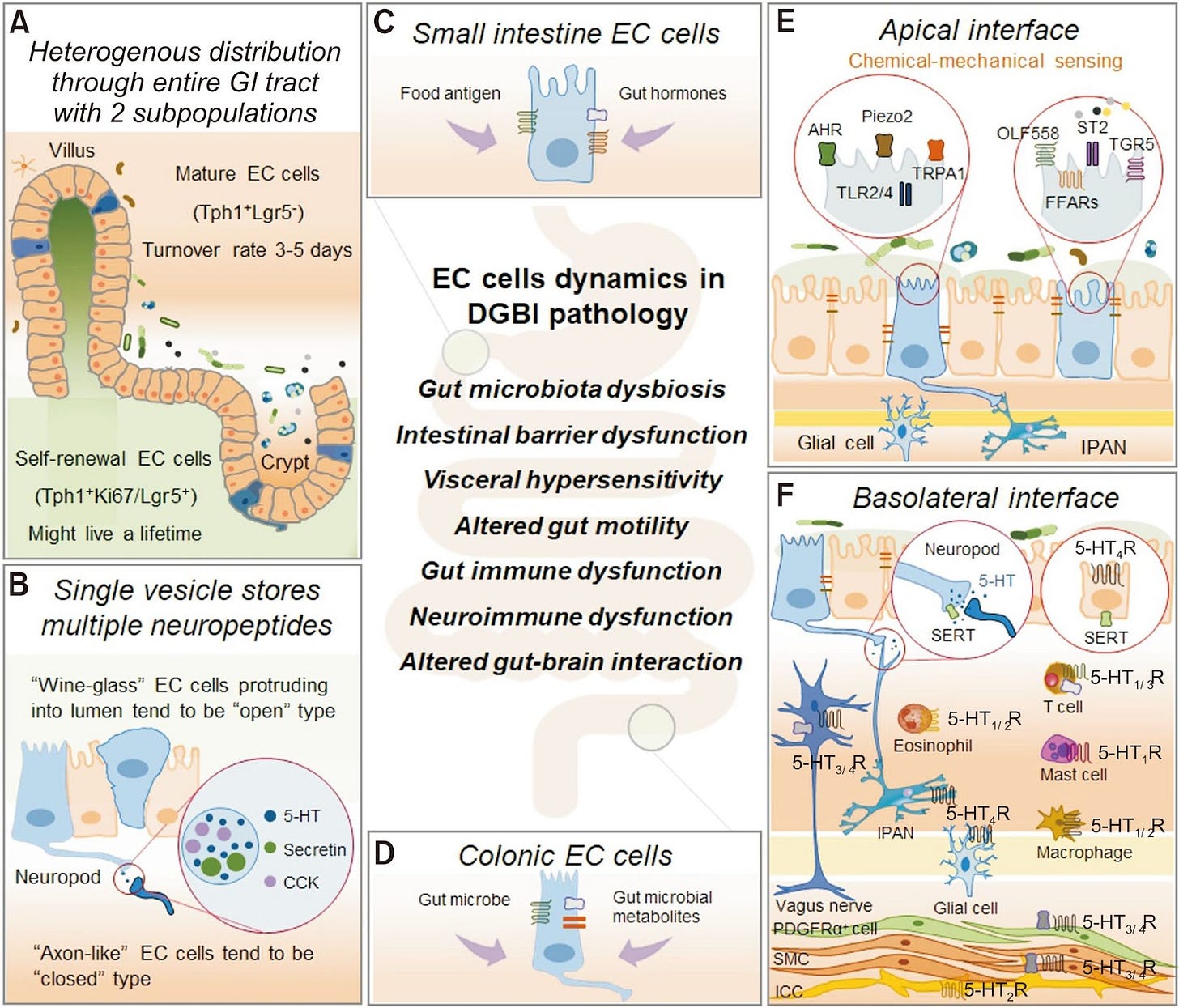
Figure 1 from Enterochromaffin Cells–Gut Microbiota Crosstalk: Underpinning the Symptoms, Pathogenesis, and Pharmacotherapy in Disorders of Gut-Brain Interaction, licensed under CC-BY-NC, illustrating the structures and dynamics of enterochromaffin cells and gut mechanisms.
Research by Kaelberer and Bohórquez has revealed a neural circuit mediated by glutamate that directly links the gut to the nodose ganglia. Synaptic links between specific enteroendocrine cells and vagal neurons were confirmed in both mouse models and in vitro organoids. Bohórquez dubbed these special gut cells “neuropods”, and some scientists credit him as being the first to discover these connections, though more study is necessary to establish the term.
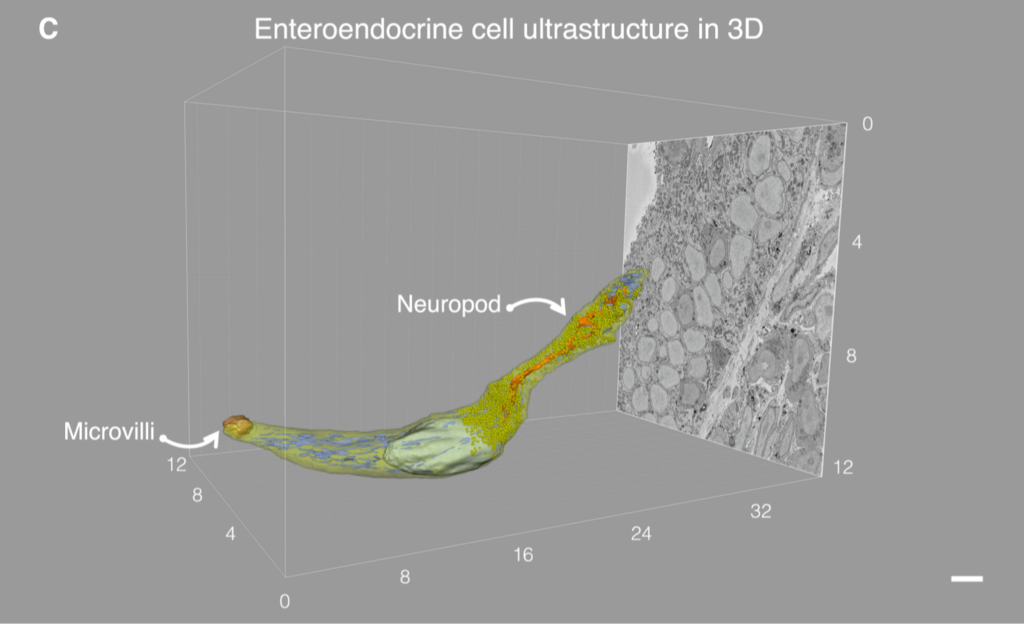
Excerpt of Figure 2 in An Enteroendocrine Cell – Enteric Glia Connection Revealed by 3D Electron Microscopy, licensed under CC-BY. This shows a 3D reconstruction of an enteroendocrine cell with an axon-like basal process, also called a neuropod, obtained via serial block face scanning electron microscopy (SBEM) of a mouse EC cell.
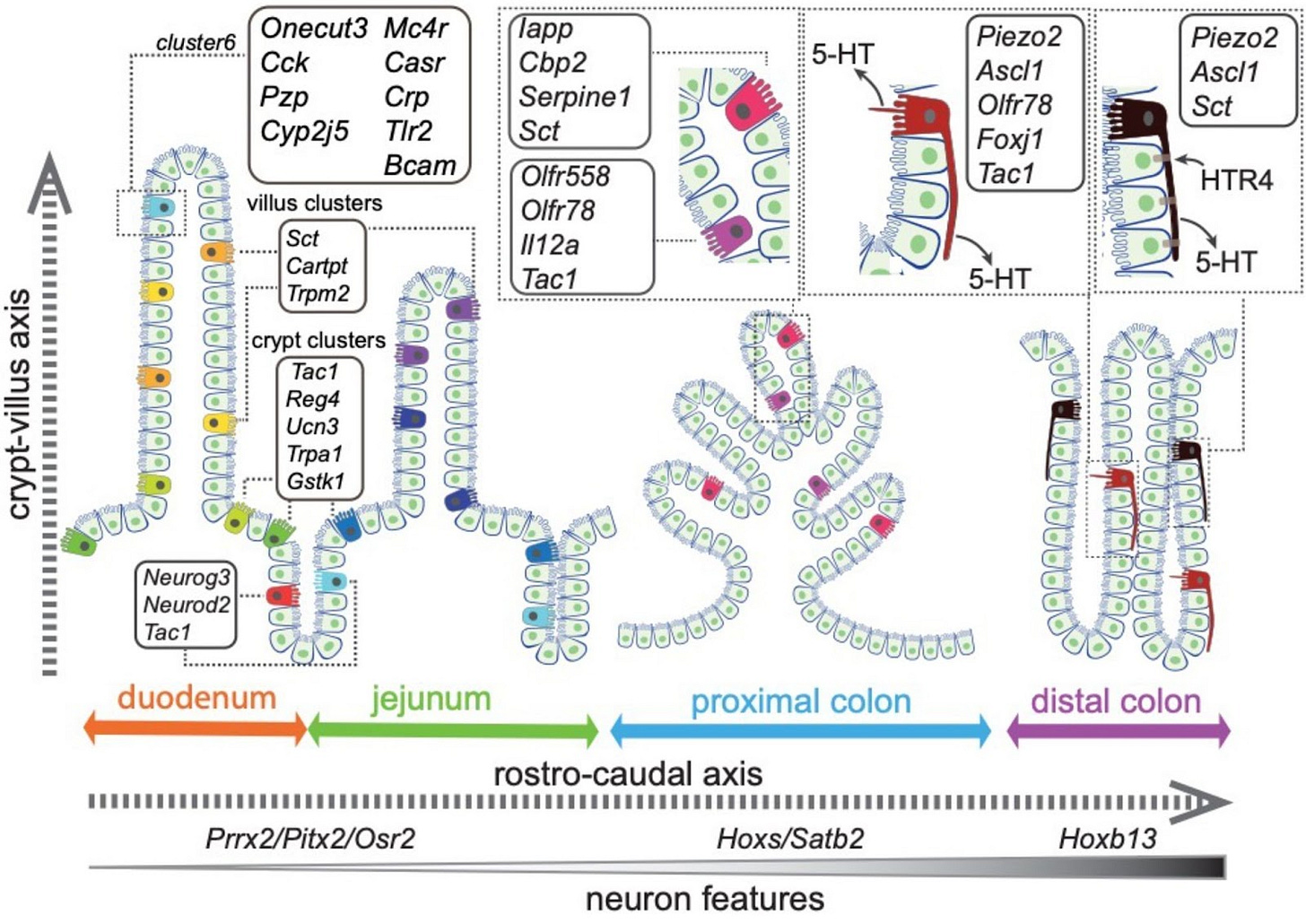
An April, 2025, paper by Yan Song and others from UC San Diego identified 14 different EC cell types in the gut, including a subset of EC cells in the colon that demonstrate “molecular and cellular characteristics reminiscent of neurons.” Specifically, the Piezo2 EC cells have transcriptome factors common to neurons as well as axon-like projections, similar to the neuropods identified by Bohóroquez. The UCSD group used mice models to confirm that these colonic EC cells affect gut motility, which is often dysregulated in people with IBS.
Figure 8 in Stratification of enterochromaffin cells by single-cell expression analysis, licensed under CC-BY. This shows different EC clusters identified in the gut, including the piezo2 cells that have neuronal characteristics and regulate motility in the colon.
In a November 2024 paper in Nature, Tongtong Wang and the Oka group at Caltech identified two distinct classes of gut neurons that separately control motility and secretion, the two primary functions of EC cells. Using a combination of transcriptomics, optogenetics, and chemogenetics–three techniques that have enabled a lot of advancements in recent neuroscience–they examined the celiac-superior mesenteric ganglia (CG-SMG), specifically looking at the sympathetic neuron population in this complex.
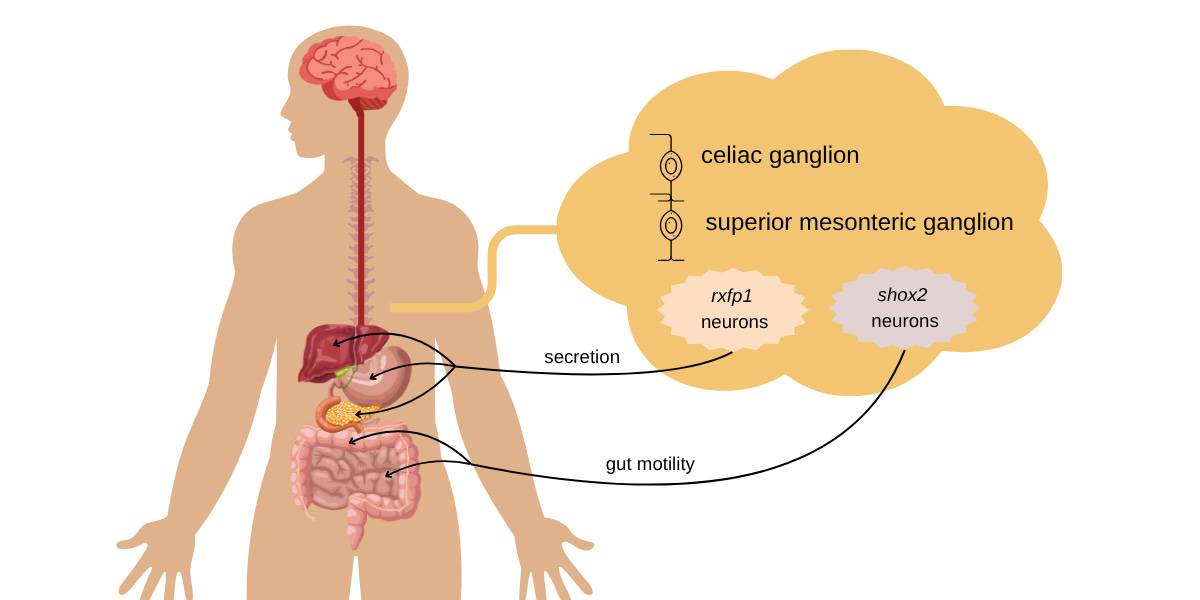
The CG-SMG complex is known to interface between the brain and the gut. The Caltech group examined 371 genes and identified two classes that are expressed by neurons, relaxin receptor type 1 (rxfp1), and homeobox gene shox2. Using genetically modified mice and optogenetic stimulation, they further determined that rxfp1 neurons down-regulate the secretion of bile and glucagon, which control digestion. The shox2 neurons down-regulate gastrointestinal transit, thereby slowing gut motility.
An illustration of the differentiation of enterochromaffin neurons in the CG-SMG complex. The rxfp1 cells innervate and control the secretory organs of the digestive system. The shox2 neurons innervate the intestinal tract and affect gut motility. Credit: Divya Srinivasan Breed
While these various findings don’t immediately point to solutions for chronic disorders like IBS, they illustrate that the digestive system contains its own complex neural circuitry, including cell types that have yet to be identified. Further work needs to be done to connect the dots between the enteric “brain”, the preganglionic neurons of the spinal cord, and the “ventral brain”, to understand the complete (and complex) circuit that regulates digestive function. My gut tells me that this will eventually lead us to more effective treatments for disorders such as mine.



Fascinating read. The connection between EC cells, neuropods, and motility makes so much sense for conditions like IBS. Feels like we’re only scratching the surface of gut neuroscience.